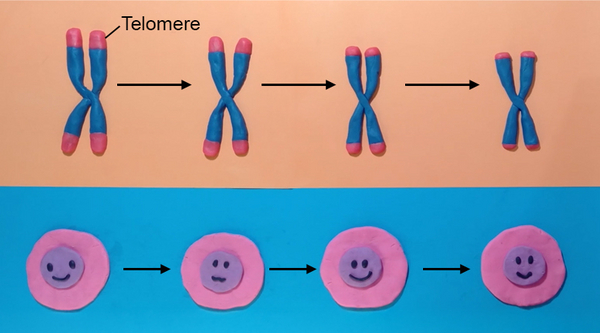
Hallmark of ageing: Telomere shortening
Protection for the ends of our genetic material
Each of us begins life as a single cell, which grows to form an entire organism. This process requires many, many cell divisions to produce the ~10,000,000,000,000 cells needed to make up our body. However, each time a cell divides, a small piece of its DNA is lost at the ends. To prevent important information (genes) from being lost, our DNA has structures called telomeres that sit at the ends, like protective caps for our genetic information. However, these also wear out over time.
Telomeres are only lengthened in some cells
In stem cells and germ cells, telomeres are renewed by the enzyme telomerase. This means that these cells can divide as often as required without losing important information. However, all other cell types do not have telomerase and have lost this ability. This is an important mechanism to prevent cells from multiplying uncontrollably and becoming cancerous.
Telomeres that are too short contribute to ageing
The disadvantage is that after many cell divisions, the telomeres become critically short and can no longer protect the DNA. The affected cell stops dividing and becomes senescent. This means that our tissues and organs can no longer regenerate properly. The "old" senescent cells also release pro-inflammatory substances, disrupt organ function and contribute to ageing.
Our age, genes and lifestyle all influence how quickly telomeres shorten. Some diseases such as dyskeratosis congenita, aplastic anaemia and Revesz syndrome are also triggered by telomeres that shorten more quickly. Patients suffer from typical signs of premature ageing, such as gray hair, hair loss, osteoporosis and insulin resistance (diabetes).
One of our research training groups in Mainz has several projects investigating mechanisms that can protect short telomeres, preventing them from making a cell senescent.
Rejuvenation by switching on telomerase in "old" cells
One approach to combat ageing is to activate telomerase in "old" cells and thus restore their telomeres. This method has already been successfully tested in model organisms: when telomerase is activated in the intestinal cells of zebrafish, age-related problems in several organ systems of the fish improve. For human patients, however, it would have to be ensured that telomerase is only activated in the intended cells to avoid increasing the risk of cancer.
Rejuvenation by removing senescent cells
Another promising approach, which is already being investigated in pilot studies with humans, is senolytics. These are drugs that specifically kill senescent cells and could thus rejuvenate ageing tissues.